Labcorp is testing for monkeypox, and a total of 200,000 vaccine doses are being distributed to states and jurisdictions as part of Biden administration's strategy to curb the spread of monkeypox
U.S. to ship 144,000 more monkeypox shots and expand testing as cases top 700
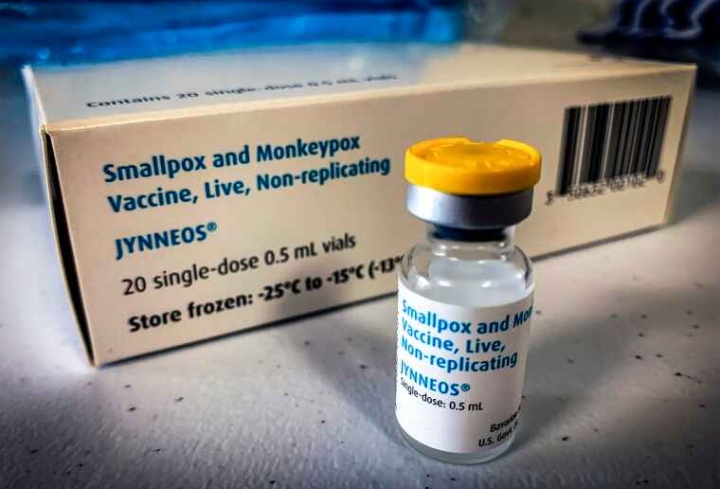

The U.S. Department of Health and Human Services (HHS) today announced that it will make an additional 144,000 doses of the JYNNEOS vaccine available to states and jurisdictions, as part of the Biden Administration’s comprehensive strategy to combat monkeypox and protect Americans most at risk from monkeypox.
These doses of JYNNEOS vaccine will begin shipping from the Strategic National Stockpile (SNS) on July 11.
The Centers for Disease Control and Prevention also announced Wednesday that Labcorp has begun testing for monkeypox at its main lab in North Carolina, which can accept samples from across the country. Labcorp expects to be able to perform up to 10,000 tests a week, the CDC said, which would double the country's testing capacity.
Four other commercial labs are expected to begin testing in the coming weeks, as well, according to HHS.
Monkeypox has spread to 54 countries and territories where the virus isn’t endemic, according to the CDC, with more than 7,200 cases globally.
"We have a very good chance at snuffing out this outbreak if we apply the knowledge and the resources that we have appropriately. I’m still hopeful that we can accelerate contact tracing and the timing of detection to quarantine, isolation and vaccination of close contacts," said Amira Albert Roess, a professor of global health and epidemiology at George Mason University.
But so far, U.S. vaccination and testing efforts have hit bottlenecks. All available vaccine appointments were quickly filled in New York City and Washington, D.C. And until Wednesday, cities and states were sending all specimens to labs in the CDC’s Laboratory Response Network to confirm infections, which led to waits for diagnoses.

 বাংলা
বাংলা  Spanish
Spanish  Arabic
Arabic  French
French  Chinese
Chinese 
